
Creativity:Transformative impact we bring to agriculture
for an innovative, sustainable, and transformative approach to modern agriculture through state-of-the-art drip irrigation solutions. Experience the future of farming with our unparalleled leadership and expertise.
The profound impact of hard water softening on agriculture irrigation

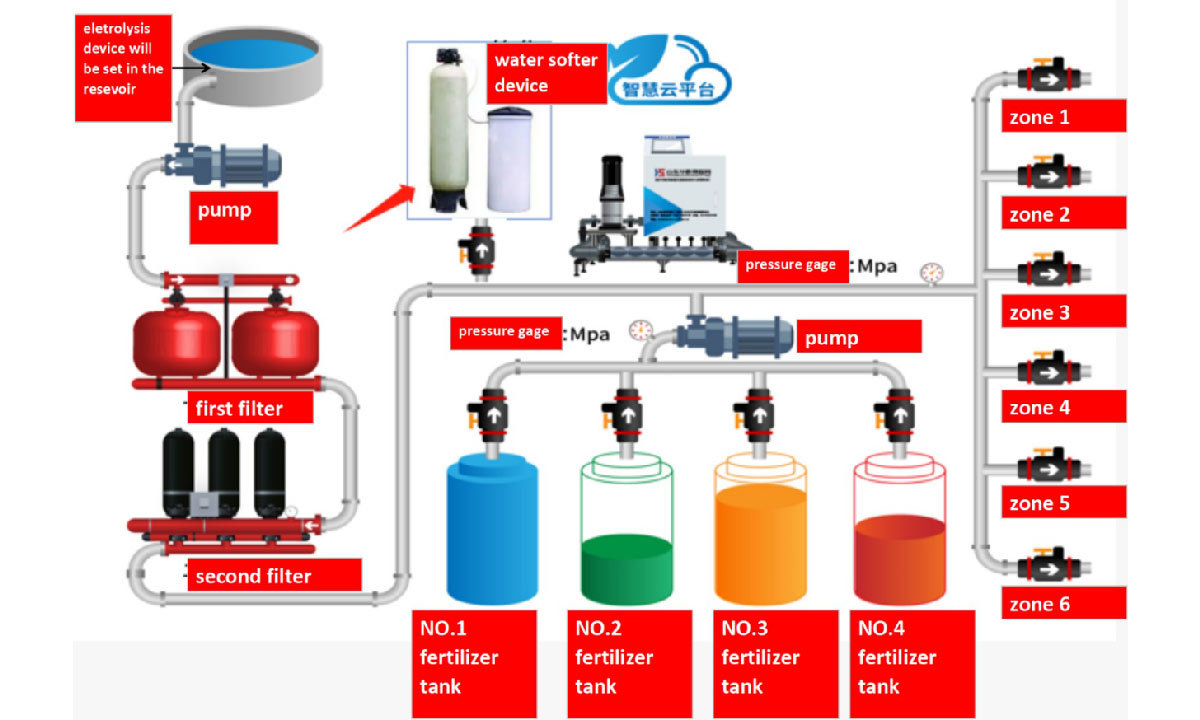
Working Process
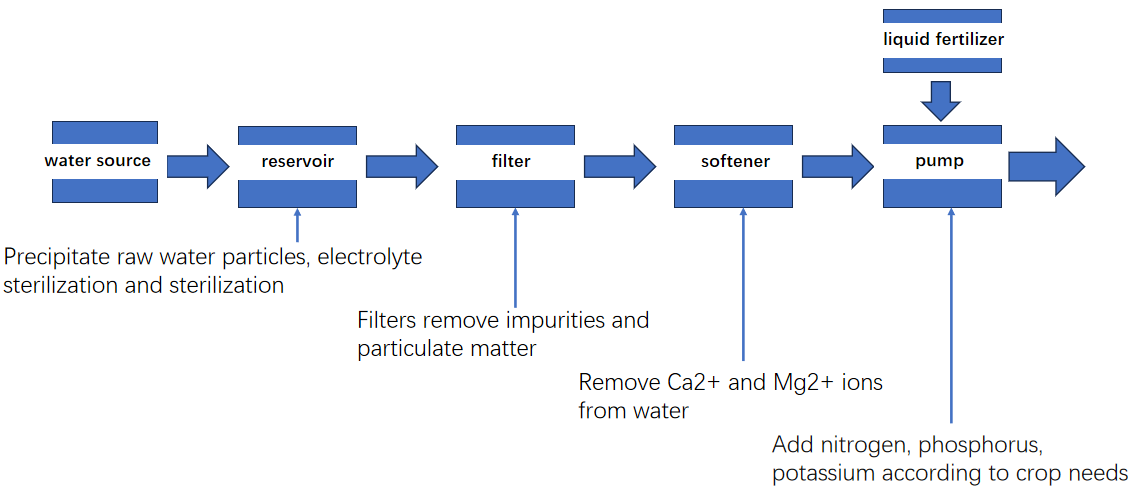
1.How to test water hardness?
Determine water hardness using total hardness test paper。
2.To soften hard water using chemical methods, lime and soda ash are used. Write down the relevant ionic equations and analyze the order in which lime and soda ash are used.?
Mg2+ + 2OH- = Mg(OH)2↓
Ca2+ + 2HCO3- + Ca2+ + 2OH- = 2CaCO3↓ + 2H2O
Mg2++2HCO3-+2Ca2++4OH- =Mg(OH)2↓+2CaCO3↓+2H2O
Ca2+ + CO32- =CaCO3↓
Use lime first, then use soda ash to remove the hardness caused by Ca2+
3.What is temporary hardness and what is permanent hardness?
The hardness caused by Ca(HCO3)2 and Mg(HCO3)2 is called temporary hardness. The hardness caused by sulfates or chlorides of calcium and magnesium is called permanent hardness.
4.Use chemical equations to explain the formation process of scale in boilers?
Ca(HCO3)2 == CaCO3↓ + CO2↑ + H2O
Mg(HCO3)2 == MgCO3↓ + CO2↑ + H2O
MgCO3 + H2O == Mg(OH)2 + CO2↑
----Hard water caused by temporary hardness can be reduced by heating and boiling..
Hard water software method
Common methods for softening hard water:
(a) Heating and boiling method:
can only reduce the temporary hardness of water, but cannot reduce the permanent hardness.
(b) Chemical softening method: (lime soda ash method)
Reduce the content of calcium and magnesium ions in water by adding chemicals.
(c)Ion exchange method:
Ion exchangers include natural or artificial zeolites, sulfonated coal and ion exchange resins, etc. In this project, we use the tree ion exchange method.
Briefly describe the principle of ion exchange method to soften hard water.
Ca2+ and Mg2+ in hard water exchange with Na+ or H+ in the ion exchange resin, reducing the concentration of Ca2+ and Mg2+ ions in the solution.
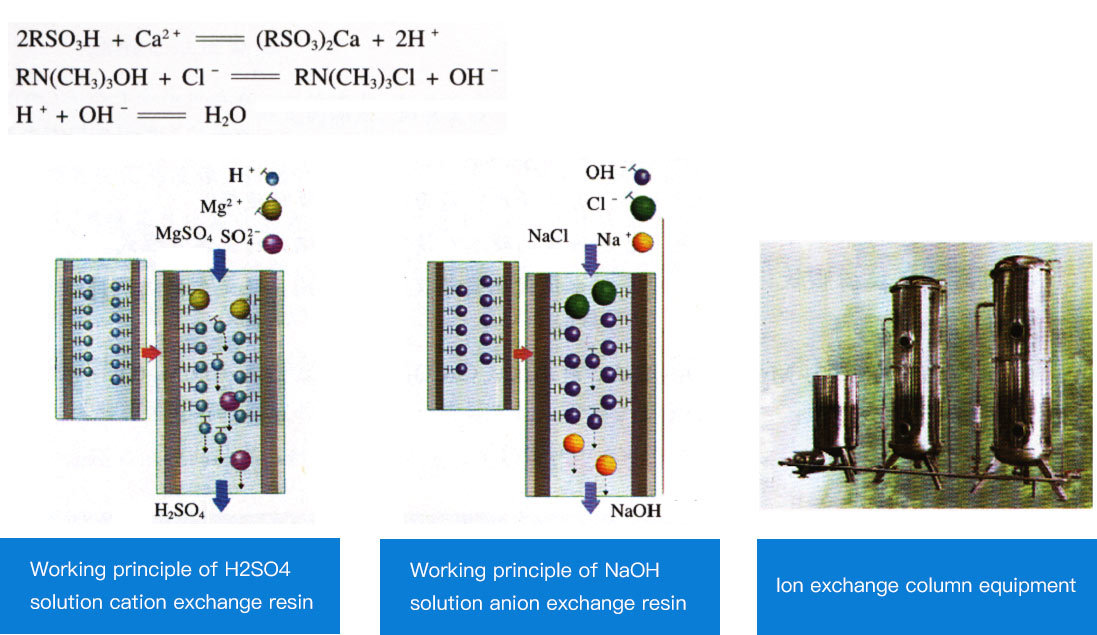
Ion exchange method
Hard water softening methods usually include chemical softening method and ion exchange method. Here we only briefly introduce the ion exchange method. Ion exchange method is a method of softening water using ion exchanger ①. Sulfonated coal (NaR) is commonly used as ion exchanger in industry. Sulfonated coal is a black granular substance that is insoluble in acid and alkali. The cations of this substance will undergo ion exchange with the cations of other substances in the solution. As shown in Figure 2-6, the ion exchange column is filled with sulfonated coal. Inject hard water from the top of the ion exchange column so that the hard water slowly flows through the sulfonated coal. When hard water passes through sulfonated coal, the Ca2+ and Mg2+ in the hard water perform ion exchange with the Na+ in the sulfonated coal, thus softening the hard water. The reaction can be expressed as follows: 2NaR+Ca2+=CaR2+2Na+ 2NaR+Mg2+=MgR2+2Na+ After all the Na+ in sulfonated coal is replaced by Ca2+ and Mg2+, the sulfonated coal loses its ability to soften hard water. However, if soaked in a sodium chloride solution with a mass fraction of 8% to 10%, CaR2 and MgR2 will exchange with Na+ to regenerate NaR, thereby restoring the ability of sulfonated coal to soften hard water. This process is called regeneration. It can be expressed as follows: CaR2+2Na+=2NaR+Ca2+ This method of softening hard water has the advantages of high quality, simple equipment, small footprint, and easy operation. Therefore, it is currently commonly used. Since ion exchange is reversible, used ion exchange resin is generally washed with an appropriate concentration of inorganic acid or alkali, and can be restored to its original state and reused. This process is called regeneration. The cation exchange resin can be eluted with dilute hydrochloric acid, dilute sulfuric acid and other solutions; the anion exchange resin can be treated with sodium hydroxide and other solutions for regeneration.
Ion exchange method
| Cationic resin (anionic resin can also be used) is used to absorb calcium and magnesium ions in the water, thereby softening the water and changing the hardness of the water. The size of the exchange resin capacity can be determined according to the incoming water quality, water volume, and water hardness. This method of softening hard water has the advantages of high quality, simple equipment, small footprint, and easy operation. At the same time, since the ion exchange effect is reversible, the used ion exchange resin is generally washed with an appropriate concentration of inorganic acid or alkali, and can be restored to its original state and reused. This process is called regeneration. The cation exchange resin can be eluted with dilute hydrochloric acid, dilute sulfuric acid and other solutions; the anion exchange resin can be treated with sodium hydroxide and other solutions for regeneration. | 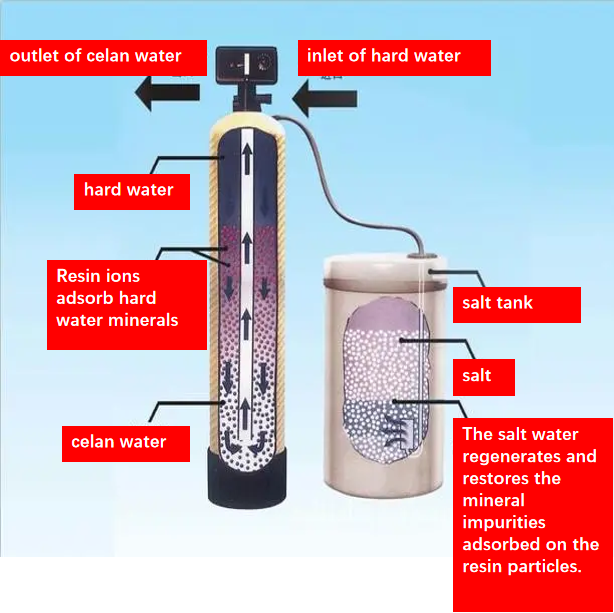 |
Resin regeneration
The electrochemical regeneration method of weakly acidic cationic resin is an effective resin regeneration technology. It uses electrochemical principles to regenerate and recover weakly acidic resins that have adsorbed a large number of cations. The steps of this method are relatively simple, easy to operate, and have high regeneration efficiency and economy.
Steps for electrochemical regeneration of weak acid cationic resin:
1. Prepare electrolyte solution:Prepare an acidic electrolyte solution of appropriate concentration, usually acidic hydrochloric acid or sulfuric acid. This solution will be used to create an electrolyte-wet environment capable of stimulating the regeneration process of weakly acidic cations.
2. Construct the electrolytic cell:Prepare an electrolytic cell that includes two electrodes (usually stainless steel electrodes or other corrosion-resistant materials). These electrodes will be immersed in an electrolyte solution to form an electrochemical system with a weakly acidic cationic resin.
3. Connect the power supply:Connect the electrodes to a DC power source that allows current to pass through the electrolyte solution and ionic resin.
4. Electrolysis process:Under the action of electric current, the acidic ions in the electrolyte will produce ion exchange in the weakly acidic cationic resin, thereby releasing the adsorbed metal ions, etc. This process, called electrochemical regeneration, gradually restores the adsorption capacity of the ionic resin.
5. Monitor and control:During the electrolysis process, parameters such as current, voltage, and ion concentration can be monitored to ensure the effectiveness and safety of the regeneration process.
6. Finishing and cleaning:When the adsorption capacity of the ionic resin gradually recovers, the electrolysis process can be stopped. Then, remove the weak acid cationic resin from the electrolyte solution and rinse the remaining electrolyte with clean water.
Electrolyzed water sterilization
| Electrolyzed water sterilization uses the principle of electrolysis to decompose water into oxygen and hydrogen through the action of electric current, and generates active oxygen species with bactericidal effect. These reactive oxygen species can destroy the cell structure of bacteria, thereby achieving the purpose of killing bacteria. The advantage of electrolyzed water sterilization is that it is a sterilization method that does not require the addition of any chemicals and does not produce harmful residues. In addition, it also has the characteristics of fast sterilization speed, good sterilization effect, and easy operation. Therefore, electrolyzed water sterilization has been widely used in food processing, drinking water treatment, medical device disinfection and other fields. |
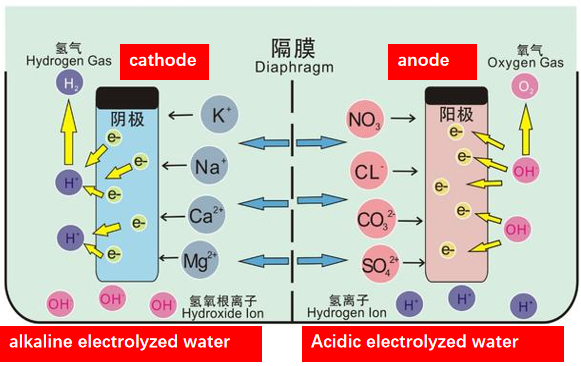 |
Resin regeneration
The electrochemical regeneration method of weakly acidic cationic resin is an effective resin regeneration technology. It uses electrochemical principles to regenerate and recover weakly acidic resins that have adsorbed a large number of cations. The steps of this method are relatively simple, easy to operate, and have high regeneration efficiency and economy.
Steps for electrochemical regeneration of weak acid cationic resin:
1. Prepare electrolyte solution:Prepare an acidic electrolyte solution of appropriate concentration, usually acidic hydrochloric acid or sulfuric acid. This solution will be used to create an electrolyte-wet environment capable of stimulating the regeneration process of weakly acidic cations.
2. Construct the electrolytic cell:Prepare an electrolytic cell that includes two electrodes (usually stainless steel electrodes or other corrosion-resistant materials). These electrodes will be immersed in an electrolyte solution to form an electrochemical system with a weakly acidic cationic resin.
3. Connect the power supply:Connect the electrodes to a DC power source that allows current to pass through the electrolyte solution and ionic resin.
4. Electrolysis process:Under the action of electric current, the acidic ions in the electrolyte will produce ion exchange in the weakly acidic cationic resin, thereby releasing the adsorbed metal ions, etc. This process, called electrochemical regeneration, gradually restores the adsorption capacity of the ionic resin.
5. Monitor and control:During the electrolysis process, parameters such as current, voltage, and ion concentration can be monitored to ensure the effectiveness and safety of the regeneration process.
6. Finishing and cleaning:When the adsorption capacity of the ionic resin gradually recovers, the electrolysis process can be stopped. Then, remove the weak acid cationic resin from the electrolyte solution and rinse the remaining electrolyte with clean water.
Automation control

Implement function
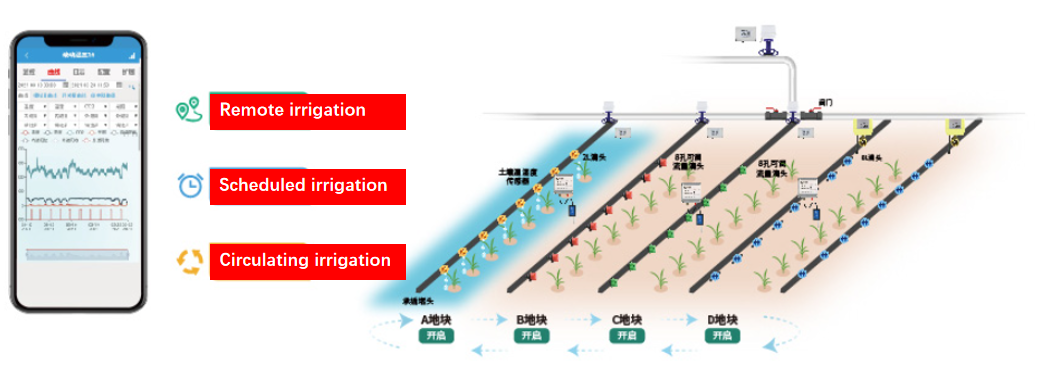
In conclusion, the profound impact of softening high pH water for agricultural irrigation cannot be overstated. It stands as a pivotal factor in fostering soil health, optimizing nutrient availability, and ultimately maximizing crop yields. This practice embodies a sustainable approach toward agriculture, ensuring long-term viability while meeting the growing demand for food production in an ever-changing world.





